
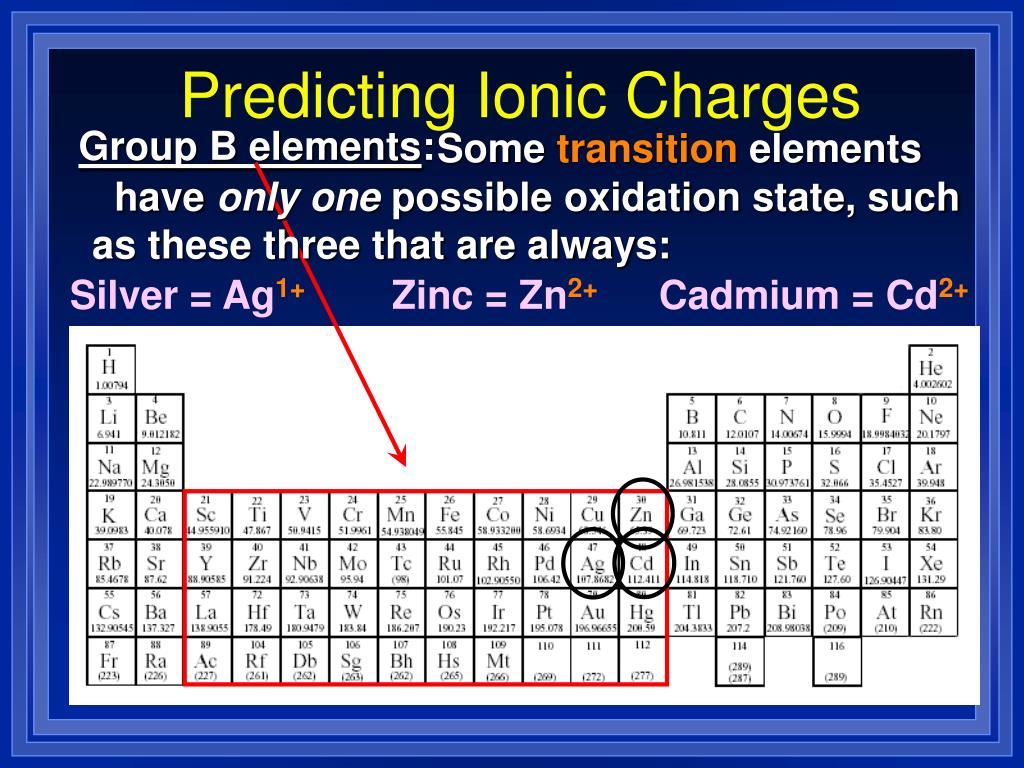
Journal of the American Chemical Society. "The Thermal Decomposition of Silver Oxide". ^ General Chemistry by Linus Pauling, 1970 Dover ed.^ US 20050050990A1, Harigae, Kenichi & Shoji, Yoshiyuki, "Fine-grain silver oxide powder", published.A Critical Survey of the Solubility Equilibria of Ag 2O". Therefore, there is no need to place parantheses. "Studies on the Hydrolysis of Metal Ions. Please note that the transition metals zinc (Zn), cadmium (Cd), and silver (Ag) do not have multiple charges. Silver 107 Metal is one of over 250 stable metallic isotopes produced by American Elements for biological and biomedical labeling, as target materials and other applications. It is both naturally occurring and produced by fission. ^ Biedermann, George Sillén, Lars Gunnar (1960). Silver 107 Metal (Silver-107) is a stable (non-radioactive) isotope of Silver. 625K subscribers Subscribe 7.

"Inorganic Chemistry" Academic Press: San Diego, 2001. This reaction does not afford appreciable amounts of silver hydroxide due to the favorable energetics for the following reaction: 2 AgOH ⟶ Ag 2 O + H 2 O : CS1 maint: multiple names: authors list ( link) Collective Volume, vol. 4, p. 547 Silver oxide can be prepared by combining aqueous solutions of silver nitrate and an alkali hydroxide. The electronic configuration of Silver will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1.Silver(I) oxide produced by reacting lithium hydroxide with a very dilute silver nitrate solution Silver-109 is composed of 47 protons, 62 neutrons, and 47 electrons. Silver-107 is composed of 47 protons, 60 neutrons, and 47 electrons. 107 Ag is the most common isotope, having a natural abundance of approximately 51. How do you write the electron configuration for Silver? Silver occurs in 2 natural isotopes: 107 Ag and 109 Ag. The electronic configuration of Silver will be 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. What is the electronic configuration of Silver 47? What is the boiling Point of Silver in Kelvin?īoiling Point of Silver in Kelvin is 2435 K. Silver-107 Ag CID 3082060 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Melting Point of Silver in Kelvin is 1234.93 K. What is the melting Point of Silver in Kelvin? What is the boiling Point of Silver?īoiling Point of Silver is 2435 K.
Silver has 47 electrons out of which 1 valence electrons are present in the 4d10 5s1 outer orbitals of atom. Explanation: Silver ions have a charge of 1+ and sulfide ions have a charge of 2. How many valence electrons does a Silver atom have? Silver was first isolated by Asia Minor in ca. Variable Charge The charge can be a different value. The element Silver was discovered by in year Before 5000 BCE. Positive charge (in the form of Zn 2+) is added to the electrolyte in the left compartment, and removed (as Cu 2+) from the right side, causing the solution in contact with the zinc to acquire a net positive charge, while a net negative charge would build up in the solution on the copper side of the cell. Exceptions: The transition metals Ag+1, Zn2+, and Cd2+ have fixed charges. It is located in group 11 and period 5 in the modern periodic table. Silver is the 47 element on the periodic table. Silver is a chemical element with the symbol Ag and atomic number 47. What is the position of Silver in the Periodic Table? Silver is a chemical element with symbol Ag and atomic number 47. To form abbreviated notation of electronic configuration, the completely filled subshells are replaced by the noble gas of the preceding period in square brackets. The abbreviated electronic configuration of Silver is 4d10 5s1. What is the abbreviated electronic configuration of Silver? The electronic configuration of Silver is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s1. What is the electronic configuration of Silver? Silver ion has a net +1 charge and nitrate ion has a net charge of -1 because the core. Silver Thermal Properties - Enthalpies and thermodynamics The silver cation (Ag+), as well as the nitrate anion (NO-3). Optical Properties of Silver Refractive IndexĪcoustic Properties of Silver Speed of Sound

Silver Heat and Conduction Properties Thermal Conductivity Since the negative charge of gram-negative bacterial cells is higher than. Refer to table below for the Electrical properties ofSilver Electrical Conductivity Yan and the team have prepared silver (Ag+) ion-selective fluorescent ONPs. Hardness of Silver - Tests to Measure of Hardness of Element Mohs Hardness Refer to below table for Silver Physical Properties Densityġ0.49 g/cm3(when liquid at m.p density is $9.32 g/cm3) The silver/silver chloride (Ag/AgCl) electrode is a widely-used electrode type for signal stimulation and detection in many groups during their Intra-body.


 0 kommentar(er)
0 kommentar(er)
